Abstract
Background Myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML) and acute myeloid leukemia (AML) are multiclonal diseases demonstrating considerable biologic, phenotypic, genetic, and clinical overlap. In recognition of this continuum, these diseases are often treated similarly. Achievement of complete remission (CR)/CR-incomplete (CRi) is deemed the major outcome associated with improved overall survival (OS), and is often used as primary endpoint in clinical AML trials, whereas red blood cell transfusion independence is often the endpoint used in MDS trials. Expert opinions questioning these endpoints are cumulating [Sekeres M, Leukemia 2019, 570; Kim N, BJH 2021, 1137; Chandhok N, Leuk Lymphoma 2022, 1], as are reports indicating that achievement of CR/CRi may not be necessary for prolonged OS in patients treated with azacitidine [Pleyer L, Annals Hematol 2014, 1825; Schuh AC, ASH 2015, P575; Pleyer L, ASH 2015, P3752; Pleyer L, ASH 2019, P3387].
All response criteria for MDS, CMML and AML require bone marrow evaluations (BME), which are only performed in ~50% of patients outside of clinical trials [Dinmohamed A, Leuk Res 2015, 177; Pleyer L, ASH 2019, P3387], indicating the real-world need for internationally accepted response endpoints that do not necessitate repeated BME. We thus introduced peripheral blood CR (PB-CR), defined as Hb ≥11.0 g/dL, PLT ≥100 and <450 G/L, ANC ≥1.0 G/L, WBC ≥1.5 and <10.0 G/L, monocytes ≤1.0 G/LPB blasts=0%, no growth factors, no transfusions). In 1441 patients treated with azacitidine, multivariate survival modelling adjusted for 17 baseline covariates, indicated that PB-CR is at least as good a predictor of OS as CR [Pleyer L, ASH 2019, P3387].
Aims To compare the predictive value of CR or CR/CRi versus PB-CR.
Methods Data from 1441 patients receiving 13971 cycles of azacitidine included in the Austrian Azacitidine Registry NCT01595295 was used for expanded analyses. The endpoints OS, time to next treatment (TTNT) and time on treatment (TOT) were defined as described previously [Pleyer L, Lancet Haematology 2021, e135]. Only data from patients who had a BME during treatment, and for whom all 17 baseline variables remaining in the final Cox model [Pleyer L, ASH 2019, P3387] were available, were analyzed (n=624).
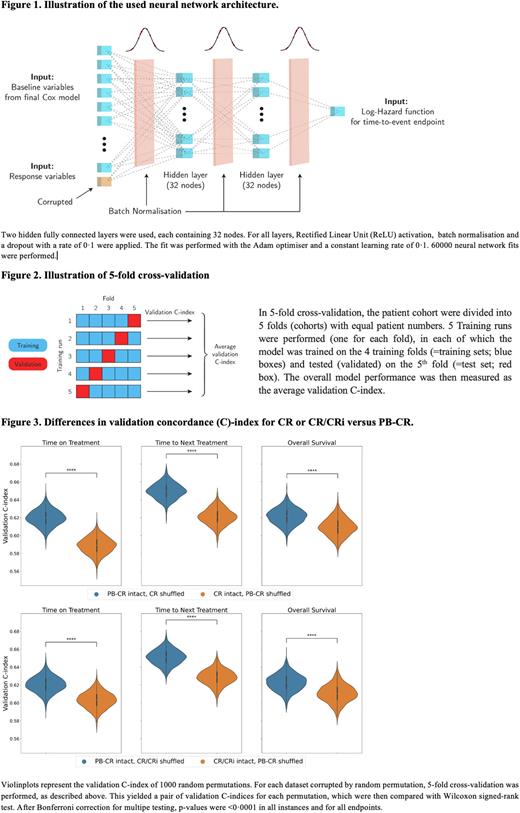
DeepSurv, a Cox proportional hazards model parametrized by a deep neural network [Katzman J, BMC Med Res Meth 2018, 24], was used to assess the predictive value of CR, CR/CRi and PB-CR. The network architecture is depicted in Fig 1.
To assess whether CR or CR/CRi has greater predictive value than PB-CR, model performance was evaluated on randomly corrupted (i.e. destroyed) data. Either CR, CR/CRi or PB-CR responses were randomly permutated 1000 times, thus removing the information content from the respective variable and allowing investigation of the resulting drop in model performance using the concordance index (C-index). The average validation C-index for each response type of the corrupted datasets was generated with 5-fold cross-validation (Fig 2), stratified by sex, age </≥75 years and diagnosis. Validation C-index pairs for each of the 1000 permutations were compared by Wilcoxon signed-rank test. Bonferroni-correction for multile testing was performed.
Results Random permutations of the PB-CR response resulted in signficantly higher performance loss than random permutations of the response types CR or CR/CRi, indicating that PB-CR has significantly more information content than either CR or CR/CRi. The C-indices with "intact PB-CR and permutated CR (or CR/CRi) were significantly higher than for "intact CR (or CR/CRi) and permutated PB-CR", with p<0·0001 for the endpoints TOT, TTNT and OS (Fig 3). Results remained consistent irrespective of the choice of inner network architectures.
Conclusions In the largest cohort of azacitidine treated patients reported to date, survival modelling with a neural Cox regression revealed a higher information content for PB-CR as compared to either CR or CR/CRi for all time-to-event endpoints analyzed. Presence of PB-CR can thus identify patients with adequate response to non-intensive therapies without repeated BM assessments.
Incorporation of PB-CR as secondary endpoint in clinical trials would be ideal to confirm these results. We aim to analyse whether these findings can be recapitulated in MDS, CMML and AML patients receiving other treatments and welcome collaborations.
Disclosures
Pleyer:BeiGene: Other: Travel/educational grant; Kite-Gilead: Other: Travel/educational grant; Novartis: Honoraria, Other: Travel/educational grant; BMS: Honoraria, Other: Travel/educational grant; AbbVie: Honoraria. Pfeilstocker:BMS: Research Funding. Stauder:BMS: Honoraria. Heibl:Novartis: Honoraria; Incyte: Honoraria; BMS: Honoraria; AOP: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria; Roche: Honoraria; Janssen Cilag: Honoraria. Sill:AbbVie: Consultancy, Honoraria; Pfizer: Honoraria; Astellas: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Hartmann:BMS: Honoraria; Amgen: Honoraria; AbbVie: Honoraria; Janssen CIlag: Honoraria. Petzer:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Sandoz: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Saegen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Schmitt:abbvie: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria. Melchardt:BMS: Honoraria; AbbVie: Honoraria. Zebisch:Novartis: Honoraria; BMS: Honoraria; Roche: Honoraria; MSD: Honoraria; Kite-Gilead: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; GEMOAB: Honoraria. Machherndl-Spandl:Jazz Pharmaceuticals: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Wolf:Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Roche: Honoraria, Research Funding; MSD: Honoraria, Research Funding; GEMOAB: Honoraria. Greil:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; MSD Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; BMS-Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding; Hoffmann - La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.